https://doi.org/10.1351/goldbook.AT07329
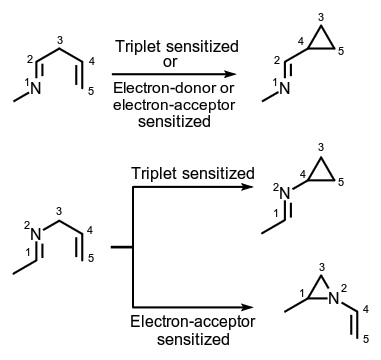
Photochemical reaction of a 1-aza-1,4-diene or a 2-aza-1,4-diene in the triplet excited state to form the corresponding cyclopropylimine.
Note: The rearrangement formally amounts to a 1,2-shift of the imino group and 'bond formation' between the C(3) and C(5) carbon atoms of the azadiene skeleton. 1-Aza-1,4-dienes also undergo the rearrangement to cyclopropylimines using electron-acceptor and electron-donor sensitizers via radical-cation and radical-anion intermediates, respectively. 2-Aza-1,4-dienes rearrange to N-vinylaziridines on irradiation using electron-acceptor sensitizers. In this instance the reaction amounts to a 1,2-shift of the alkene unit and 'bond formation' between the C(1) and C(3) carbon atoms of the azadiene skeleton.