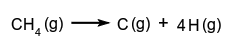https://doi.org/10.1351/goldbook.B00701
The average value of the gas-phase bond dissociation energies (usually at a temperature of 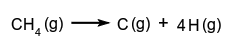 Tabulated bond energies are generally values of bond energies averaged over a number of selected typical chemical species containing that type of bond.
Tabulated bond energies are generally values of bond energies averaged over a number of selected typical chemical species containing that type of bond.