https://doi.org/10.1351/goldbook.C00806
The electrically neutral species H2C: and its derivatives, in which the carbon is covalently bonded to two univalent groups of any kind or a divalent group and bears two nonbonding electrons, which may be spin-paired (singlet state) or spin-non-paired (triplet state). In systematic name formation, carbene is the name of the parent hydride :CH2 hence, the name dichlorocarbene for :CCl2. However, names for acyclic and cyclic hydrocarbons containing one or more divalent carbon atoms are derived from the name of the corresponding all-
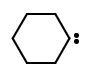
Subclasses of carbenes include acyl carbenes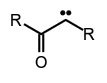 , imidoyl carbenes,
, imidoyl carbenes, 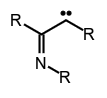 and vinyl carbenes.
and vinyl carbenes.
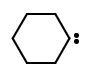
Subclasses of carbenes include acyl carbenes
 , imidoyl carbenes,
, imidoyl carbenes,  and vinyl carbenes.
and vinyl carbenes.