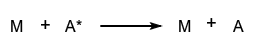https://doi.org/10.1351/goldbook.C01164
The collision efficiency, or de-energization efficiency, is defined by: 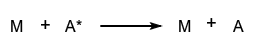 and
and