https://doi.org/10.1351/goldbook.E01948
A molecular rearrangement that involves the formation of a σ-bond between the termini of a fully conjugated linear π-electron system (or a linear fragment of a π-electron system) and a decrease by one in the number of π-bonds, or the reverse of that process. For example: 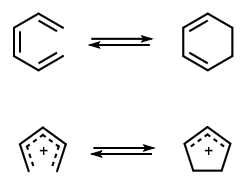 The stereochemistry of such a process is termed 'conrotatory' or antarafacial if the substituents at the interacting termini of the conjugated system both rotate in the same sense, e.g.
The stereochemistry of such a process is termed 'conrotatory' or antarafacial if the substituents at the interacting termini of the conjugated system both rotate in the same sense, e.g. 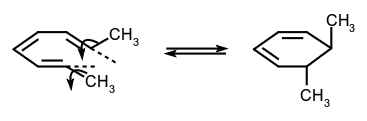 or 'disrotatory' (or suprafacial) if one terminus rotates in a clockwise and the other in a counter-clockwise sense, e.g.
or 'disrotatory' (or suprafacial) if one terminus rotates in a clockwise and the other in a counter-clockwise sense, e.g. 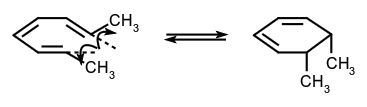

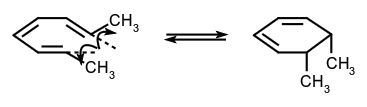
See also: pericyclic reaction