https://doi.org/10.1351/goldbook.R05186
This idea may be expressed loosely as: the more reactive a reagent is, the less selective it is. Consider two substrates 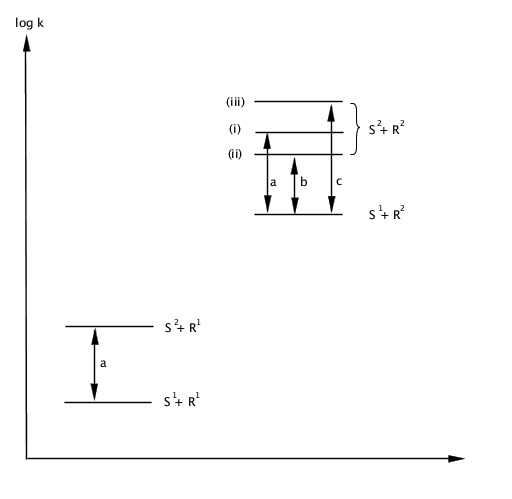 reactive than
reactive than
With the positions of (

With the positions of (

See: selectivity