https://doi.org/10.1351/goldbook.08786
Molecule or a part of a molecule, of which structure may be represented as a system of alternating single and multiple bonds, in which, eventually, a multiple bond can be replaced by an atom with a pair of non-bonding electrons or an atom carrying a negative charge.
Notes:
- Examples of conjugated systems are conjugated polymers and molecule species whose formulas drawn in terms of the valence bond theory are as follows:
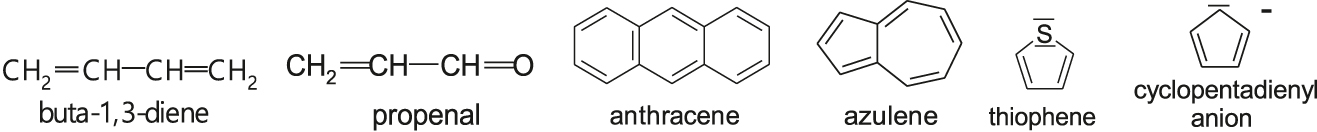
- Alternating arrangement of the single and multiple bonds allows the delocalization of electrons, which stabilizes conjugated systems. The most effective stabilization show planary cyclic systems with
- Due to the delocalization of electrons a formula drawn in terms of the valence bond theory does not fully describe the structure of a conjugated species, which actually is a combination of two or more limiting or intermediate resonance structures that are in a dynamic equilibrium. The molecular orbital theory which directly provides extended molecular orbitals spread over all contributing atoms allows better understanding of the electronic and optical properties of a conjugated system.