https://doi.org/10.1351/goldbook.C01024
A concept related to rate of appearance, particularly applicable to the progress in one direction only of component reaction steps in a complex system or to the progress in one direction of reactions in a system at dynamic equilibrium (in which there are no observable concentration changes with time). Chemical flux is a derivative with respect to time, and has the dimensions of amount of substance per unit volume transformed per unit time. The sum of all the chemical fluxes leading to destruction of B is designated the 'total chemical flux out of B' (symbol 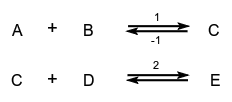 the total chemical flux into C is caused by the single reaction (1):
the total chemical flux into C is caused by the single reaction (1):