https://doi.org/10.1351/goldbook.I03199
Minimum energy required to eject an electron out of a neutral atom or molecule in its ground state. The adiabatic ionization energy refers to the formation of the molecular ion in its ground vibrational state and the vertical ionization energy applies to the transition to the molecular ion without change in geometry. This quantity was formerly called ionization potential. The second ionization energy of an atom is the energy required to eject the second electron from the atom (energy for the process 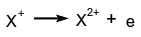 ).
).
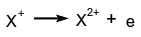 ).
).