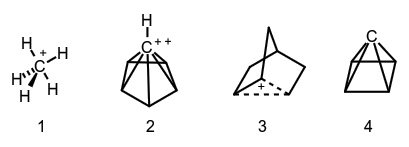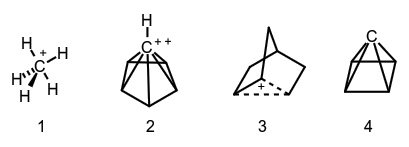https://doi.org/10.1351/goldbook.NT07084
The structure of molecules or molecular ions that escapes description in terms of conventional rules of valency and stereochemistry. Nonclassical structures are characteristic of carbonium ions with hypercoordinated (see hypercoordination) carbon atoms, e.g., methanium ion 1, pyramidal dication C6H62+ 2 (isomeric to benzene dication), and the molecular species whose structure cannot be adequately represented by the equilibrium (2-norbornyl cation, 3) or resonance of two or more classical structures. From the stereochemical point of view, those structures are assigned to the nonclassical type for which all tetracoordinate carbon bonds extend into a single hemisphere, i.e., the valence angle of a carbon atom is greater than 180°. A hypothetical example is pyramidane, 4, the structure of which corresponds to a local minimum on the C5H4 potential energy surface.