https://doi.org/10.1351/goldbook.P04476
A Penning gas mixture consists of a rare gas containing impurity atoms possibly at very low concentrations. The impurity atoms have an ionization potential 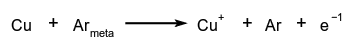 In a glow discharge, this results in an increase of the the ionization coefficient (Townsend first coefficient), a decrease in breakdown potential and a lowering of the cathode fall potential. The magnetic Penning effect describes the increase of the ionization probability of gas in a low pressure electrical discharge resulting from the helical (spiral) movement of electrons in a magnetic field placed normal to the anode-cathode electrical field.
In a glow discharge, this results in an increase of the the ionization coefficient (Townsend first coefficient), a decrease in breakdown potential and a lowering of the cathode fall potential. The magnetic Penning effect describes the increase of the ionization probability of gas in a low pressure electrical discharge resulting from the helical (spiral) movement of electrons in a magnetic field placed normal to the anode-cathode electrical field.